Hydrogen is an ideal energy source. It is easy to produce using renewable energy sources and is available in large quantities. No pollutants result from H2-Industries’s processes. Nothing is burned. Nothing is polluted. Nothing is damaged.
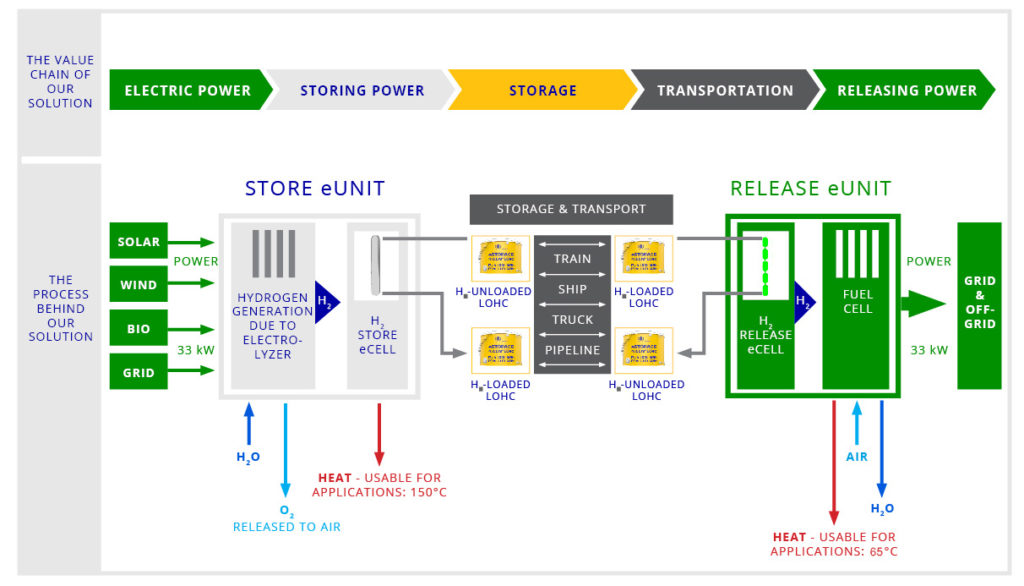
H2-Industries technology is revolutionary and portends a huge advance in the electricity market.it Hydrogen has become a safe energy source from creation, storage, and transport to release.
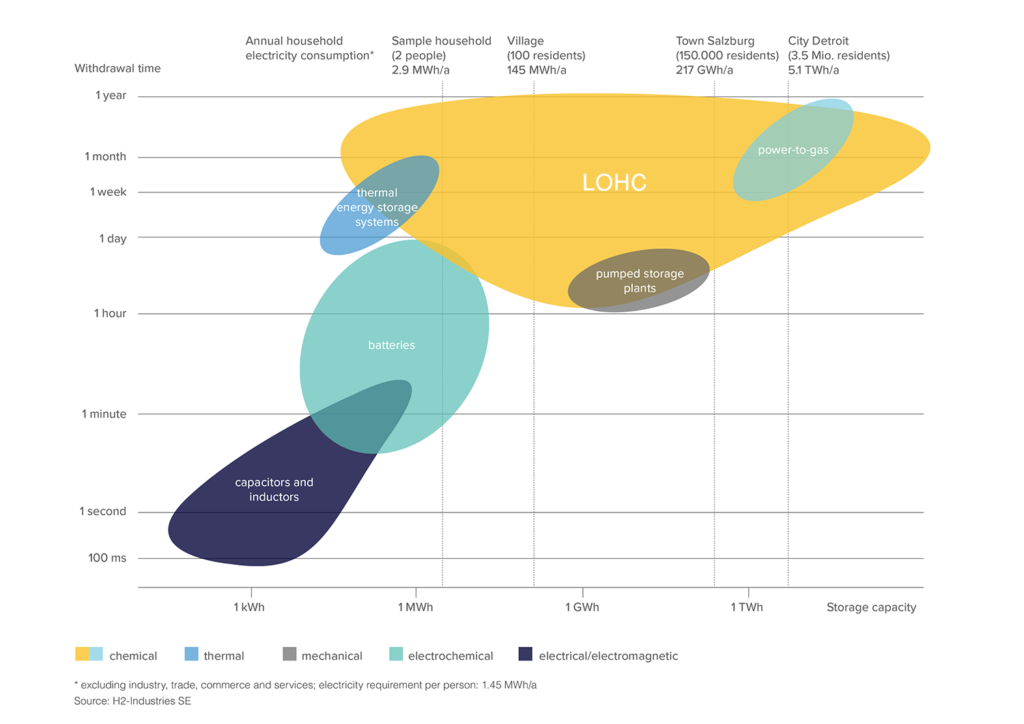
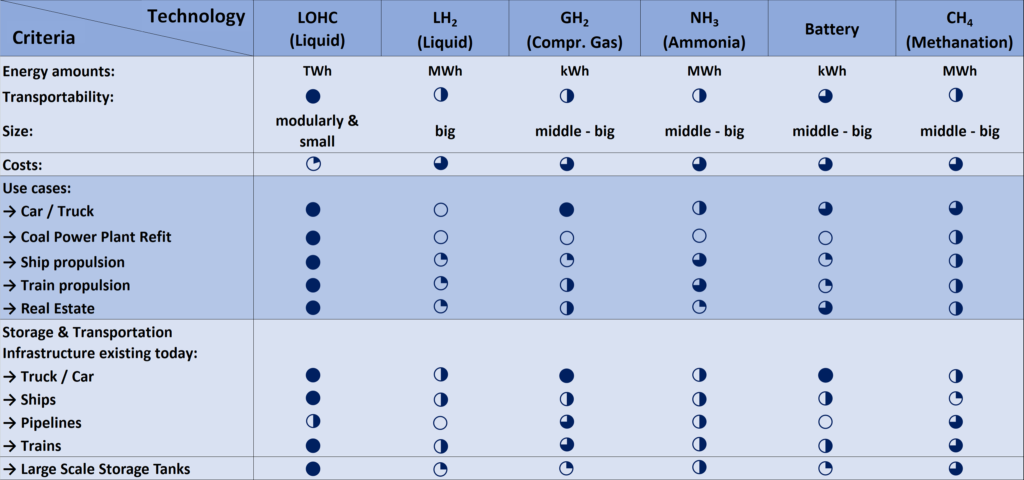
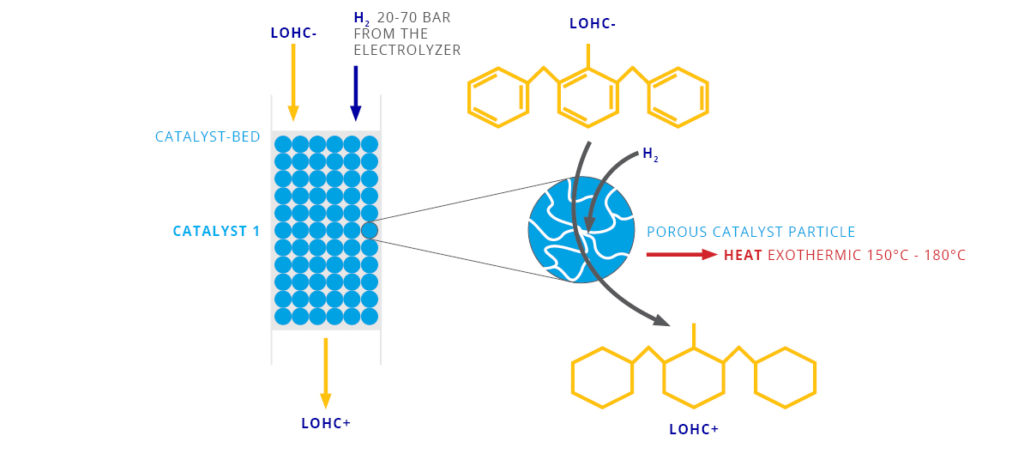
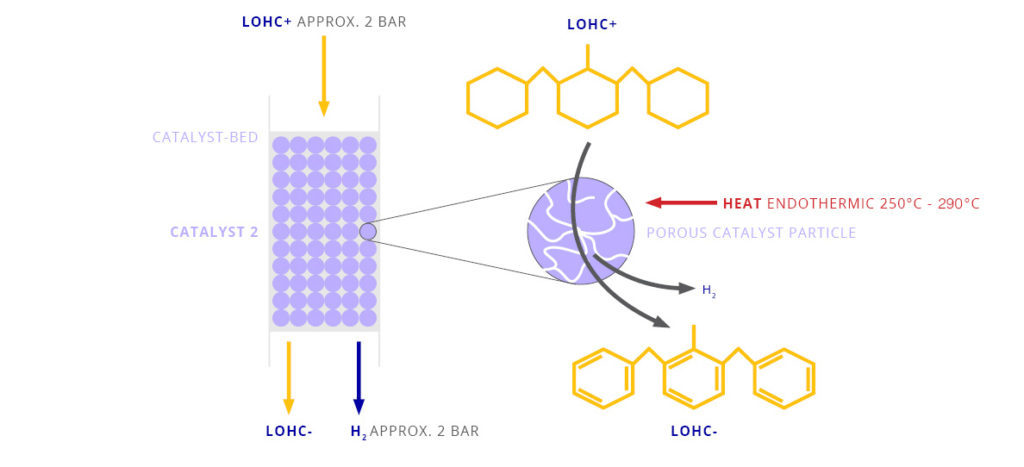
The LOHC technology is based on liquid carriers, that bind hydrogen chemically. Within H2’s process, stored hydrogen is not volatile and cannot self-discharge. The LOHC can be charged and discharged only in combination with a catalyst, infusing and releasing hydrogen, as often as needed making it remarkably cost effective