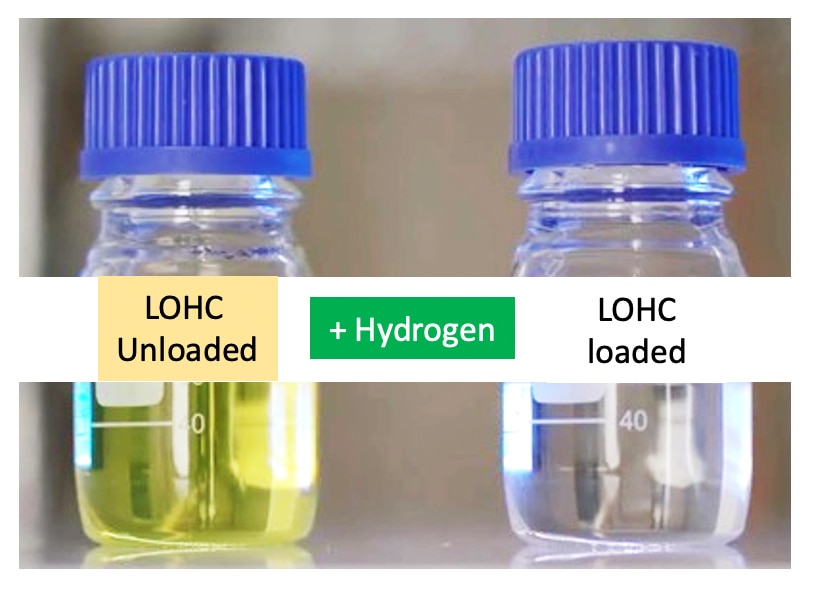
How does LOHC work?
With pressures between 30 – 50 bar and catalysts specially developed for this application, the LOHC can be hydrogenated, i.e. hydrogen can be chemically bound. The resulting hydrogenated LOHC + can then be handled using the known infrastructure for fuels such as gasoline and diesel. The hydrogenation process is exothermic. The waste heat developed in this way can be used in other processes and thus increases the overall system efficiency.
If the hydrogen is needed again, for example in chemical process plants, the steel industry or to supply fuel cells in order to use electrical energy, it can be extracted again from the LOHC +.
In order to dehydrate the LOHC +, i.e. to release the hydrogen from the liquid again, the LOHC + passes through a dehydrogenation reactor, which contains the catalyst required for this process. In contrast to hydrogenation, dehydrogenation is an endothermic reaction. The necessary energy must therefore be added and can, for example, be made available within the system by using the hydrogen itself or provided by other, external heat sources.
The dehydrogenated LOHC- can now be returned to the location of the hydrogenation and reloaded with hydrogen. The cycle is closed. The LOHC itself is not consumed, but reused many times over. The service life is also increased by the possibility of purification as soon as this becomes necessary after various (de) hydrogenation cycles.